COVID-19 Vaccine Development: The Jump to Lightspeed
Posted 16 February 2021To solve this conundrum, the first step is to have a clear overview of the development stages of a vaccine ensuring its quality, safety and effectiveness, as shown below.
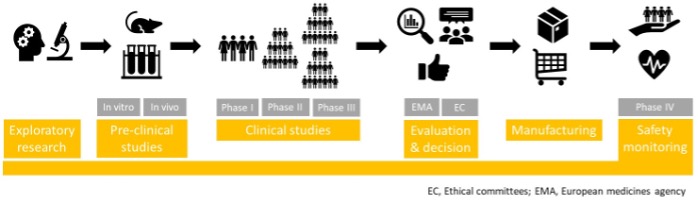
1) The exploratory stage consists of basic research screening the pathogen for potential target antigens, determining the most suitable vaccine type and formulation, and investigating the manufacturing process in detail.
2) Once promising vaccine candidates are identified, they are narrowed down in pre-clinical studies which are conducted in vitro (tissue/cell-culture systems) and/or in vivo (animal models) to evaluate their safety (including the best administration route and dosing) and immunogenicity.
3) At this point, regulatory authorities (RA) need to make sure that the vaccine’s quality profile is satisfying so that it can safely move on to clinical studies, which include 3 phases:
- human pharmacology studies (phase I): based on 20-100 healthy volunteers, establishing the vaccine’s safety and immunogenicity as expected from laboratory results;
- therapeutic exploratory studies (phase II): based on >100 volunteers (including subjects more at risk of developing the disease or not), confirming the vaccine’s safety and immunogenicity, and identifying the optimal vaccine dose, route of administration, schedule of immunization and common side effects; and
- clinical efficacy and safety studies (phase III): based on >1000 volunteers presenting the disease or not, confirming the vaccine’s immunogenicity and evaluating its capacity to protect against the infection/disease development compared to a placebo/alternative treatment. Less common side effects are also identified at this stage.
4-5) After a successful phase III trial, a meticulous evaluation of the vaccine’s test results and manufacturing conditions (presented in a “marketing authorization” application) is performed by RAs to confirm the safety and quality of vaccine batches intended for market release. Ultimately, a vaccine will only be approved for manufacturing if its benefits are considered to outweigh its risks.
6) Finally, following approval, safety monitoring/pharmacovigilance (= phase IV trials) is continuously performed to ensure that any possible risk is detected and managed as fast as possible. In addition, every new batch of vaccine is tested for quality and safety before its commercial release.